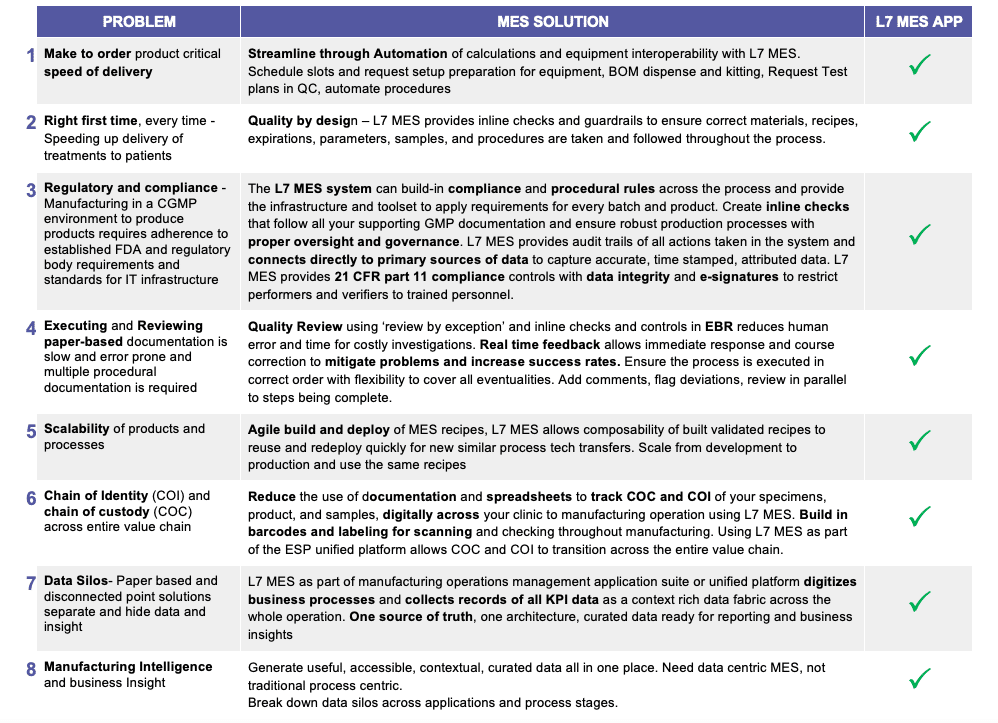
L7 | CHATS
product update
L7|ESP Manufacturing Execution System (MES) – Sample Plans, Sample Points, and Dynamic Execution Plans
by Kevin McMahon, Precision Therapeutics Solutions Lead | posted on June 27, 2023
Introduction
Following on from the L7 MES app introduction, let us have a deeper dive into the features that set the L7 MES app as the right choice for pharmaceutical manufacturing. One of our guiding principles in designing the L7 MES app was to build the core regulatory compliance requirements all cGMP processes must adhere to, out of the box, and additionally, how to support the timely dispositioning of manufactured finished products.

Sample Plans
Sample plans are an essential element of quality control and testing for the disposition and release of products to any market. Each market and governing body require potentially different release testing to verify the product was manufactured in a controlled state. Methods are developed to determine the safety, quality, identity, potency, and purity of the produced batch.
Managing the methods associated with sample types and the collection and storage conditions of the samples is paramount to the testing and sample retain process. The sample plan documents these set requirements associated with products to ensure correct testing for release is carried out for each batch.
- Sample plans help ensure that the manufacturing process is consistent and reproducible.
- Sample plans help detect any potential problems early in the manufacturing process, which can help prevent costly delays and recalls.
- Sample plans help ensure that the final product is safe and effective for human use.
How the L7 MES application adds industry knowledge to our solution
Sample Plan
Build your QC samples, retain and in-process samples into one, easy-to-navigate dashboard of your product specific sample plan. Built into your master batch recipe (MBR) and extendable for different approved sample plans and ad-hoc customer requests for any given MBR. One process recipe, multiple products.
- Add sample points with multiple sample types anywhere in your process.
- Print labels pre-batch and reprint as needed during execution.
- Keep track of samples taken and by whom.
- Reconcile labels printed to labels used easily after batch is complete.
- Use barcodes to scan into EBR during collection and watch sample plan update with relevant information.
- Review and generate document of recorded samples.
- Each sample is a child of the batch.
- Chain of Custody and Chain of Identity is tracked throughout the sample-test lifecycle.
- Testing conducted on same system in LIMS app for in-process testing and QC testing.
- Use legacy systems with the L7 MES app to document sampling in EBR.
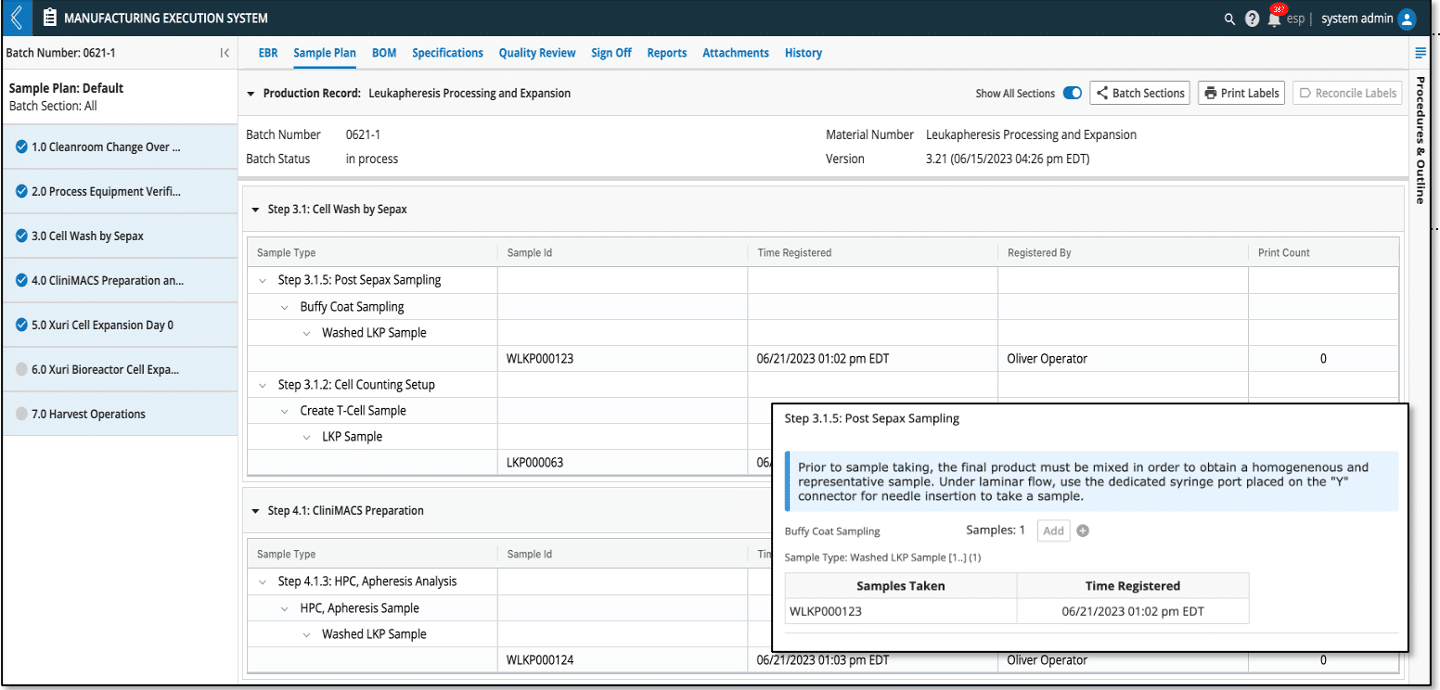
Figure 1: Sample plan dashboard and corresponding Sample point for adding samples in EBR.
Dynamic Execution Plans
Approved Sample Plans can be created for any MBR to overwrite the default settings and expand the product choice on each master batch record, choose the combination at batch start.
- Multiple approved Sample plans easily created and ready to use for every product.
- Customer requests for additional samples prior to execution become a simple task.
- Centralized control over sample plans.
- Reduce the number of MBRs required to execute assorted products on the same process train.
- Quality assurance can confirm correct plans are chosen prior to execution.
- Choose your plan and print the correct labels for every variation in sampling and testing requirements.
- Annual or biannual characterization, requalification samples are dynamically added to your EBR.
- Full control of product execution without red lines and messy comments.
- Documented record of plans used in batch production.
- End to End Chain of Custody and Chain of Identity from batch to QC testing.
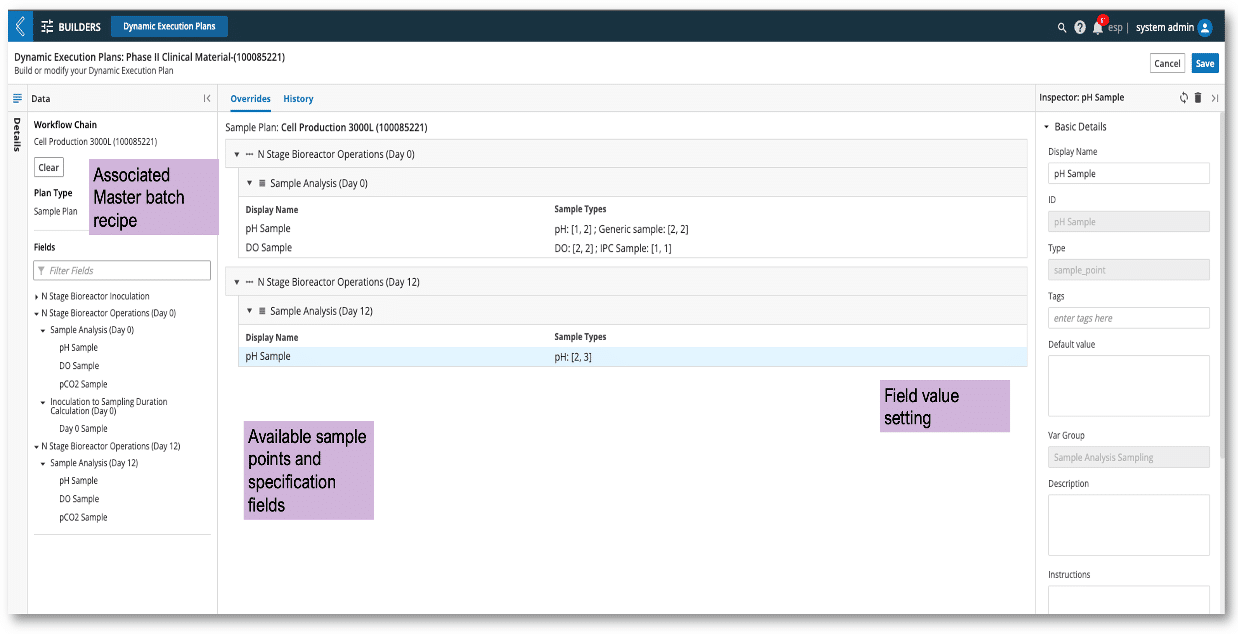
Figure 2: Dynamic Execution Plan pulls available sample point fields to create a new adjustable sample plan.
The L7 MES application, as part of the L7 Enterprise Science Platform (L7|ESP), provides a holistic manufacturing operations management experience. We are continually improving and adding additional applications to our unified platform to really tackle the needs of our customers and to provide the end-to-end infrastructure required to really accelerate and normalize biotech and C> businesses to their full potential. The L7 MES app system is designed for developing and then progressing to cGMP production seamlessly, with data and process automation at the heart of the process development, CMC work, and tech transfer to commercial production. We are cGMP-ready from the start but are flexible enough to support the early-stage process development, capturing and curating your data resource across your journey to clinical and commercial production.
Standard functionality and features of the L7 MES app for your CMC, Development, Tech transfer, and CGMP needs: