L7|ESP® for CDMOs
navigating CDMO core challenges
In the dynamic world of pharmaceuticals, CDMOs play a crucial role. Yet, as the demand for rapid technology transfers and increased manufacturing capacity intensifies, CDMOs are under increasing pressure to deliver novel products swiftly and efficiently. Understanding and overcoming these challenges is not just about survival—it’s about thriving in a competitive market.
Seamless Collaboration
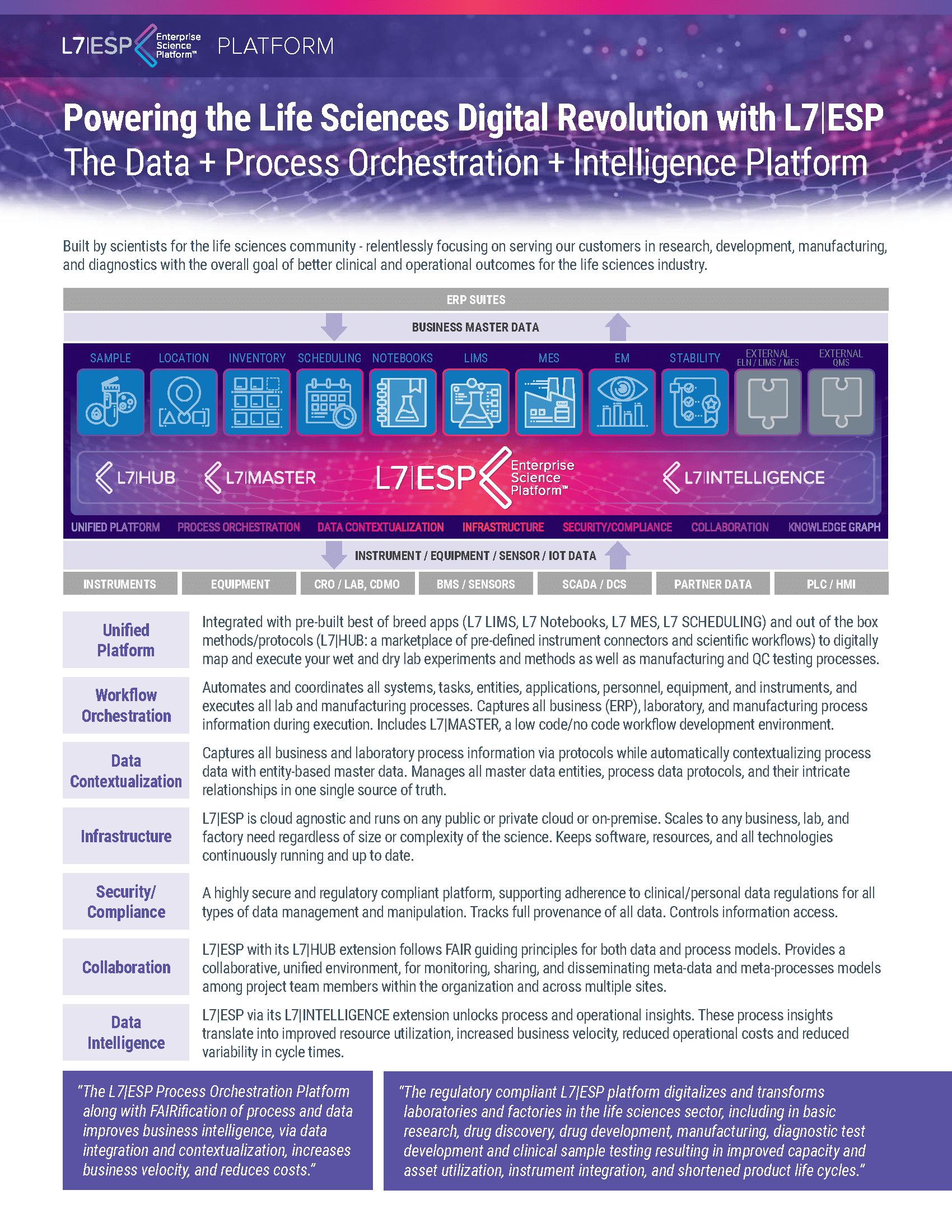
L7|ESP facilitates unmatched collaboration across all stages of drug development and manufacturing. By enabling digital integration, the platform ensures that all team members, regardless of physical location, can collaborate efficiently, share insights instantly, and make informed decisions quickly. This interconnectedness is crucial for maintaining the pace required to meet client demands and regulatory standards.
Data Exchange
With its robust data management capabilities, L7|ESP ensures a seamless and secure exchange of critical information across different phases of the product lifecycle. The platform’s ability to manage and synchronize data not only streamlines workflows but also protects data integrity, enhancing the reliability of every output. This comprehensive data exchange supports CDMOs in achieving faster technology transfers and more effective scale-up processes.
Visibility Solutions
L7|ESP provides comprehensive visibility across the entire product lifecycle, from initial development to final manufacturing. This visibility is critical to maintaining control over the complex processes and compliance requirements inherent in CDMO operations. By centralizing and contextualizing data, L7|ESP delivers detailed insights and traceability, empowering CDMOs to anticipate issues, optimize resources, and maintain continuous improvement in their operational practices.
empowering CDMOs with L7|ESP
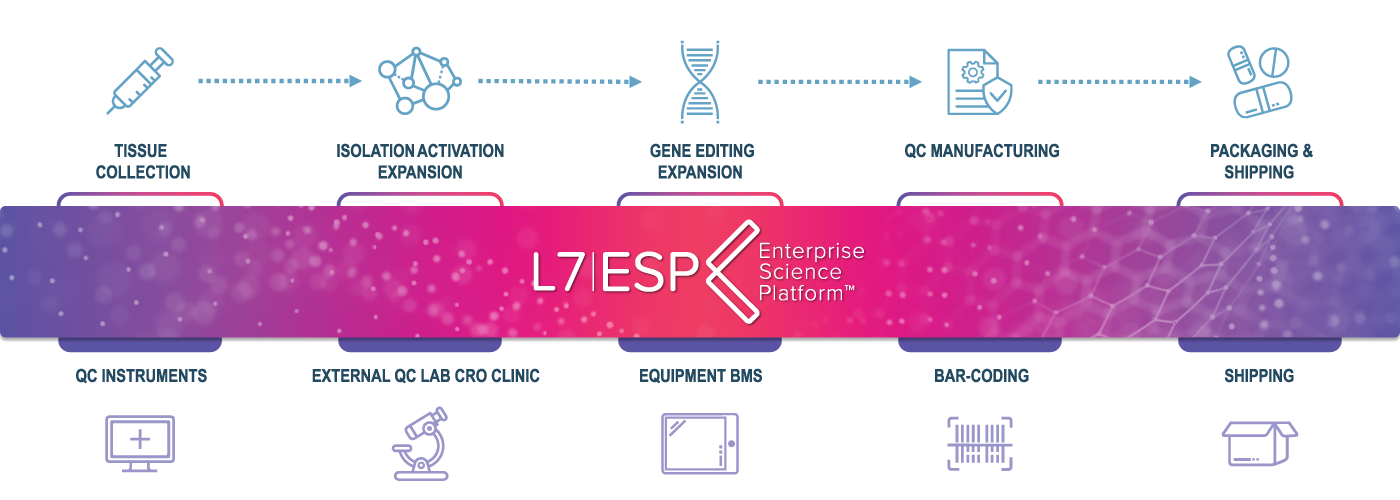
Tailored for CDMOs, our unified platform, L7|ESP, centralizes all critical functions from development to manufacturing, production, and shipping, ensuring seamless integration and consistent data management. It harnesses the power of real-time analytics to deliver actionable insights, enabling faster and more informed decision-making that optimizes both production and quality.
With automated compliance tools, L7|ESP simplifies adherence to complex regulatory standards, ensuring each product meets stringent quality requirements. Adaptable to varying project demands and scalable to accommodate growth, L7|ESP enhances efficiency and ensures high-quality outcomes, making it an essential tool in driving operational excellence and facilitating market readiness.
the transformative benefits of L7|ESP for CDMOs
L7|ESP stands as a catalyst for efficiency and quality in the complex environment of CDMOs. By integrating advanced technology, such as Industrial Internet of Things (IIoT), cloud connectivity, AI, and machine learning, and its data and process orchestration capabilities, L7|ESP delivers substantial benefits tailored to the needs of CDMOs:
Enhanced Operational Efficiency
L7|ESP optimizes the entire manufacturing process, from initial development stages to final production and shipping. Its streamlined workflows minimize manual interventions and reduce the likelihood of human error, accelerating production timelines and reducing operational costs. With L7|ESP, CDMOs can achieve higher throughput without compromising quality, making each project more profitable and time-efficient.
Scalability for Future Growth
As CDMOs grow and take on more complex projects, the ability to scale operations effectively becomes essential. L7|ESP is designed to scale seamlessly with your operations, accommodating increased production demands without the need for proportional increases in overhead or resources. This scalability ensures that CDMOs can expand their client base and handle larger contracts efficiently.
Consistency and Quality Assurance
At the heart of L7|ESP’s capabilities is its ability to maintain stringent quality controls across all stages of manufacturing. The platform ensures consistent adherence to product specifications and regulatory standards, critical for CDMOs aiming to build trust with their clients. By automating quality checks and integrating real-time monitoring, L7|ESP provides a robust framework for ensuring product integrity and compliance.
Data-Driven Decision Making
With the integration of real-time analytics, L7|ESP turns data into one of your most valuable assets. The platform’s analytics capabilities allow CDMOs to anticipate challenges, optimize resource allocation, and improve overall decision-making. This proactive approach reduces downtime and enhances the ability to respond swiftly to market demands.
what customers are saying
resources
Dive deeper and see how L7|ESP can transform your operations: