L7 | CHATS
product update
Introducing L7|ESP Manufacturing Execution System (MES) – A Unified Platform approach to Manufacturing Operations Management
by Kevin McMahon, Precision Therapeutics Solutions Lead | posted on April 14, 2023
Introduction
Previously, we have looked at the major pain points affecting precision therapeutics manufacturing capabilities. The reliance on paper-based processes and spreadsheets for operations can cause major bottlenecks in production and release of C> products to patients. Current solutions don’t have the flexibility or composability needed in this industry. The earlier the correct digital strategy is employed, the sooner efficiencies and quality by design can be applied to your processes and thus alleviate a paper or a siloed digital approach.
How L7 approached the MES Solution
We first looked at all the current capabilities in L7|ESP that could be leveraged to build a holistic digital solution to using paper and spreadsheets in manufacturing. At the core of digitization is the desire to automate the governance and procedures that keep the process in control and, ultimately, the patient safe.
Additionally, managing the data resources accurately and cleanly, that are created during development, CMC, and manufacturing, drives innovation in the manufacture of today’s and tomorrow’s novel C> treatments.
With L7|ESP’s ability to build complex business workflows and automate procedures, we expanded the functionality by looking at:
- Customers CGMP manufacturing requirements and constraints.
- Focusing on digitizing where “people meet process”.
- Keep the solution familiar and easily adopted.
- Replace paper and spreadsheet transactions with a digital solution.
- Build quality by design (QbD) and real-time feedback in the solution.
- Build as an extension of ESP that works seamlessly with all ESP applications.
- Elevate the UX/UI for our Customers.
- Speed up disposition of product by providing all the elements needed.
- How to work across product development to CGMP in one solution
Let’s look at a typical cleanroom manufacturing system in Figure 1. We can see many departments interacting, passing information, documents, materials, equipment, samples, and products through preparation, production, testing, release, and shipment. Represented by the separate and colored business process flow paths.
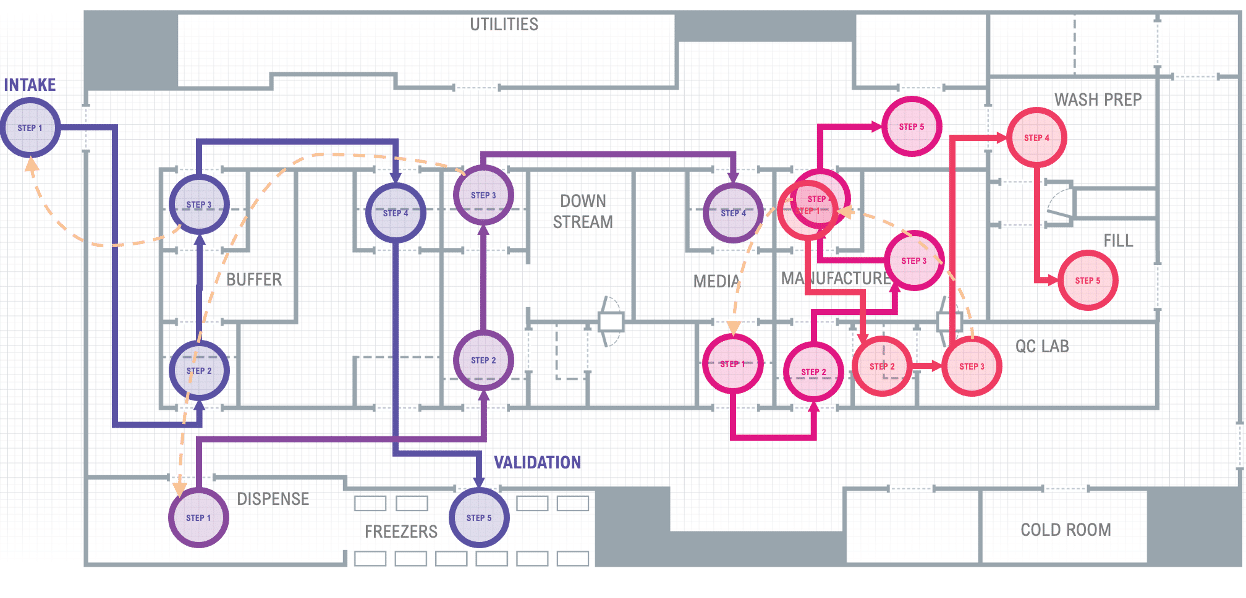
Figure 1 Typical Cleanroom Manufacturing System with multiple departmental touchpoints
The Precision Therapeutics manufacturing relies heavily on paper batch records, forms, and spreadsheets to fill the functional gaps manufacturing operations require for day-to-day operation. Traditional MES is very rigid and siloed from other systems and equipment, leaving operations still relying on forms and spreadsheets to function.
L7 MES Provides Value-add functionality for CMC to CGMP Manufacturing
- Easy navigation at all levels of the process.
- Familiar layout of a batch record with automatic numbering for batch section, step, and sub step.
- Quality by design, build-in limits and exceptions to automate your inline checks and get real-time feedback.
- Interoperability with equipment and systems to connect to primary source of data.
- Sequential execution control with role and individual-based permissions.
- On screen tabs for real-time in-process data and quality oversight with a click.
- 21 CFR part 11 compliance with data integrity and electronic signatures designed for your CGMP purposes.
- Communicate effectively with images and tables to support written instructions.
- Add multiple comments on every entry in execution and after in quality review.
- Easy retrieval of relevant digital standard operating procedures (SOP) during execution, connect to electronic document management system for current documents.
- Attach files or images of setup, associated printouts, or data files at appropriate steps or to the batch.
- Run complex calculations and generate real-time trends and graphs to support decision making.
- Connect to your Pi-Historian to get discreet data points from tags or across a period for tables and time-lapse.
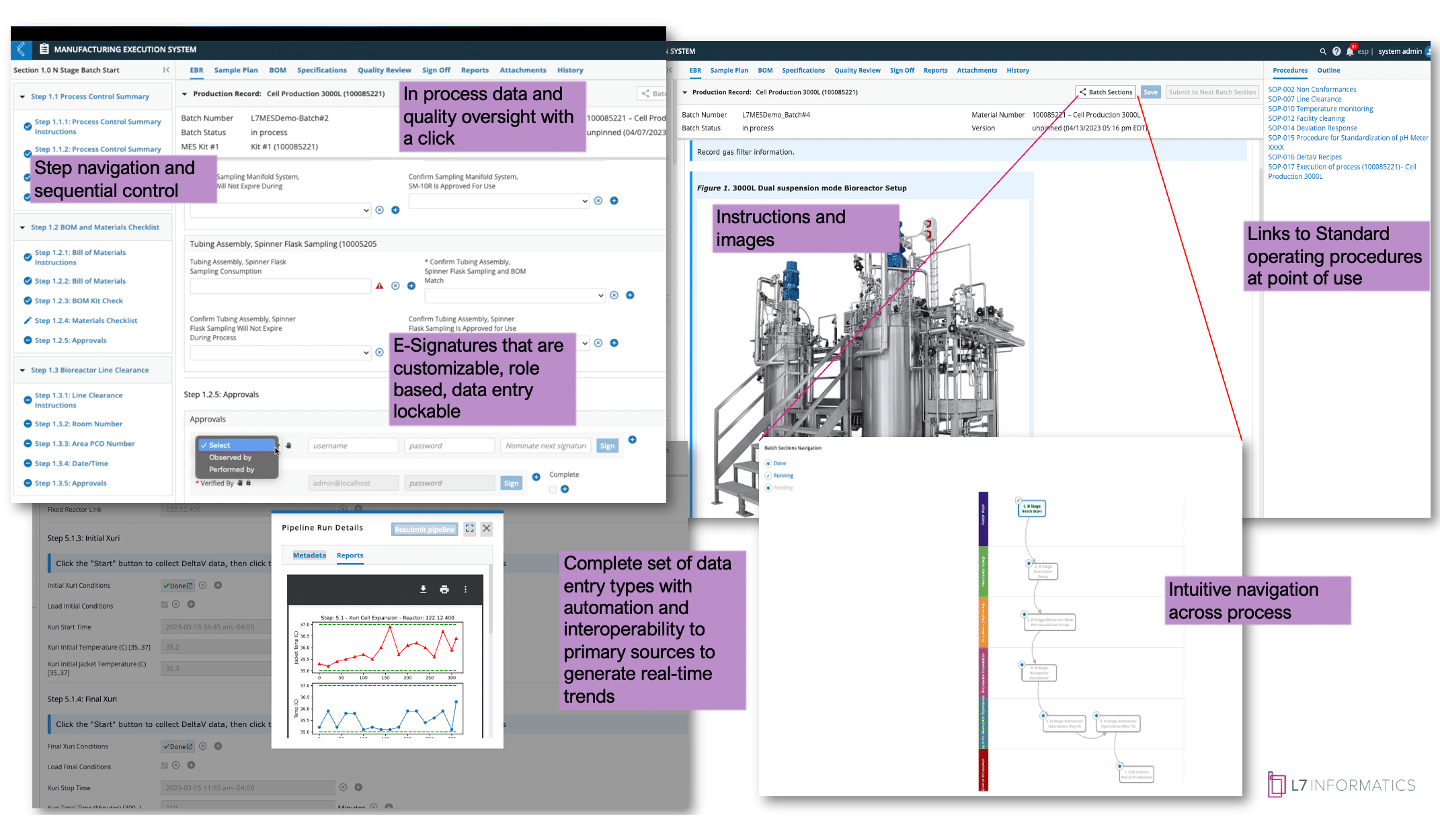
Figure 2: L7 MES Electronic Batch Record View
Figure 2. presents the L7 MES app with an overview of the electronic batch record (EBR) view that an operator would use to execute the batch. The EBR has multiple features, as described above, that cover automating your procedures in a digital format while capturing the execution data in a way that is familiar to someone who is used to paper. The added real-time checks reduce the chances of entry error and ensure your procedural controls are applied during day shift at 5:00 PM or at 5:00 AM on a weekend shift, just before handover.
The L7|MES system is designed for developing and then progressing to CGMP production seamlessly. We are CGMP-ready out of the box and provide the flexibility to support the early-stage process development, capturing and curating your data resource across your journey to clinical and commercial production.
We have touched on our EBR functionality in the MES system, and we will delve deeper into the exciting features we bring to the MES solution table in my next blogs. How L7 MES seamlessly interacts with the rest of L7 Enterprise Science Platform (ESP) and how that builds in functionality that will make manufacturing connected and traceable across a manufacturing system such as in figure 1.
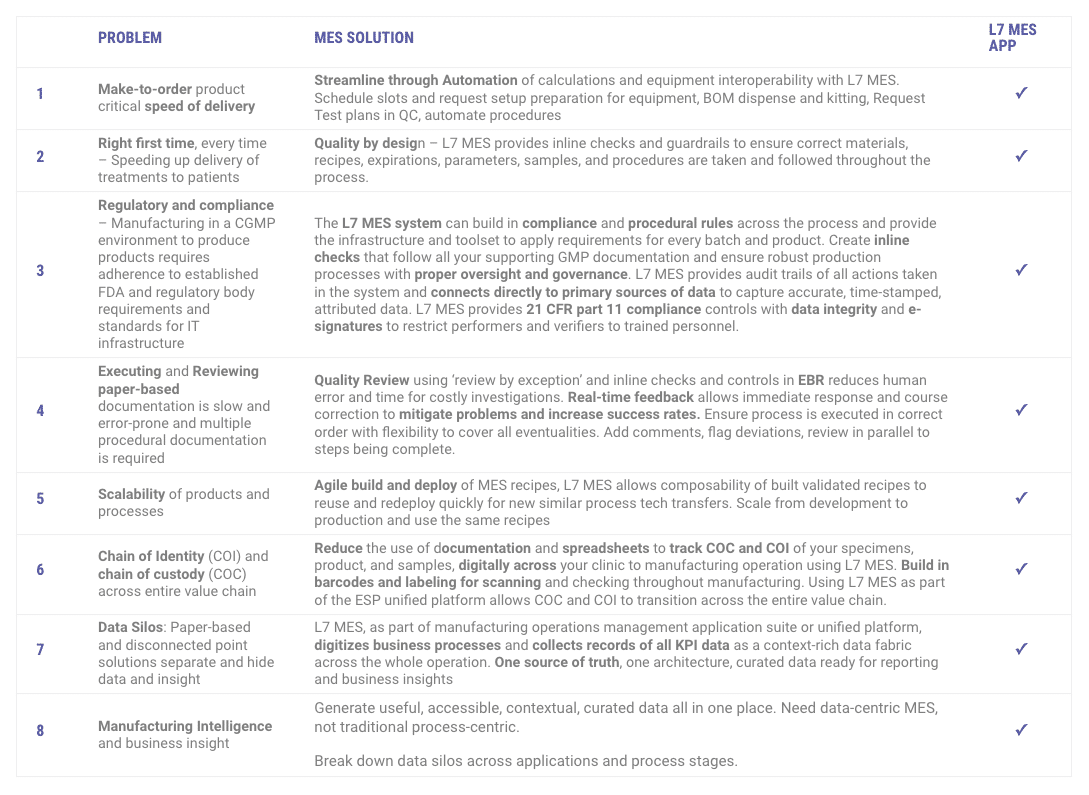
Standard functionality and features of the L7 MES app for your CMC, Development, Tech Transfer, and CGMP needs: