L7 | CHATS
thought leadership
Powering the Life Sciences Digital Revolution with L7|ESP
by Brigitte Ganter, Ph.D. | posted on April 30, 2024
The Data + Process Orchestration + Intelligence Platform
Life science processes, be it in research, drug discovery, development, manufacturing, or diagnostic test development and execution, have grown in complexity and scale over the last decade, not the least because the industry is facing ongoing pressures such as ROI (return of investment) challenges and/or loss of exclusivity on key assets.
This rapid growth has resulted in disconnected and sub-optimal laboratory process management systems and the lack of a unified data strategy that would provide the necessary laboratory and business process insights with the potential to shorten the overall product life cycle. To overcome these challenges, the industry quickly adopted innovative high-throughput technologies, heavily invested in modernizing data infrastructure, and – more recently – in Artificial Intelligence (AI) capabilities to derive insights from the rapidly growing volumes of data. To derive high-value insights from an AI data model requires contextualized data as input. Otherwise, the saying “garbage in, garbage out” holds true. Adding AI capabilities on top of uncontextualized and siloed data returns sub-optimal results, while data volumes and costs of storage are continuing to grow without an equivalent ROI expected from these new IT investments.
Fractured Processes and Data Can Be a Threat to Achieving ROI Goals
Siloed data systems across labs and factories not only result in high maintenance and integration IT costs but also a loss in process and business knowledge that could be translated into increased business velocity and shortened product life cycles (aka faster drugs to market).
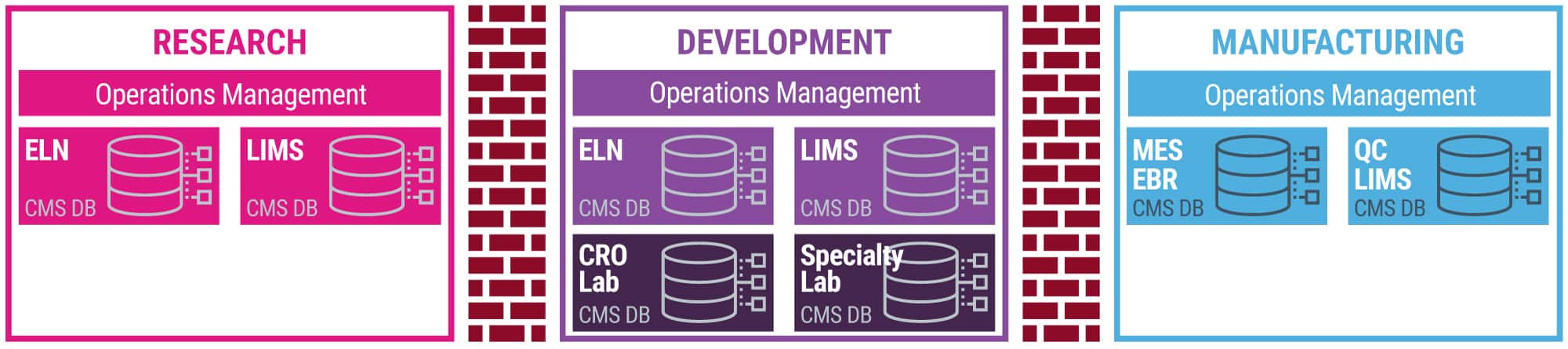
- Fractured vendor and home-grown systems (i.e., different information management systems that manage the laboratory processes | ELN, LIMS, EBR, MES, QC LIMS) across different groups and departments in drug discovery and development, and manufacturing.
- Legacy systems are not able to handle the advances in data sciences.
- No foundational, company-wide data strategy to make data and processes findable, shareable, accessible, and reusable between labs and scientists within and across departments, organizations, and multiple sites. This results in a lack of visibility and transparency on research, laboratory, and business data across labs and onerous and expensive regulatory submissions.
- High IT costs to maintain the various laboratory information management systems and data infrastructure.
The Solution is L7|ESP – Purpose Built for the Life Sciences Based on Industry 4.0 Principles
The Unified Platform L7|ESP is built based on best practices of industry 4.0 principles (The Fourth Industrial Revolution, Klaus Schwab, 2017), which reinvented how life science businesses conduct their research and develop, manufacture, and distribute their products. Technologies, such as Industrial Internet of Things (IIoT), cloud connectivity, AI, and machine learning (ML), are nowadays becoming deeply woven into the drug discovery, development, and manufacturing processes. Taking advantage of this unified and integrated approach to bringing new drugs to market results in highly connected and informed people, products, factories, and assets. Combining accuracy and speed of 4.0 tools with the creativity and talent of people yields is a win/win situation for both the workforce and the bottom line of a company. Drug research, development, and manufacturing operations become more efficient and productive, with individual teams and team members being relieved of low-value and repetitive operational tasks, providing them with the opportunity to efficiently collaborate instead with smart technologies embedded in the evolving technological landscape and the AI-powered future of their work.
Meet Today’s and Tomorrow’s Organizational Needs of Maximizing the Total Return on Information Technology Investment
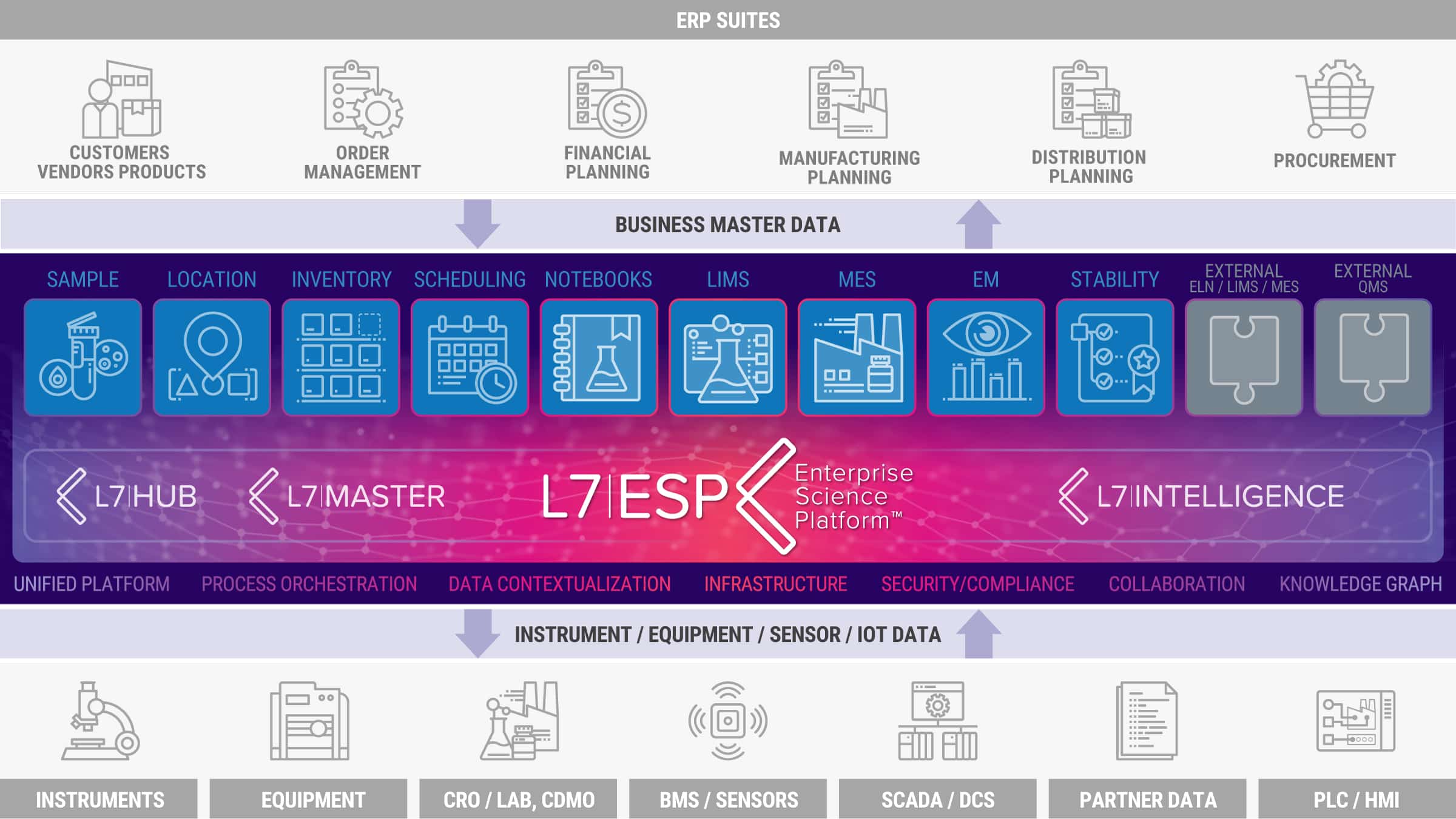
The L7 Informatics’ Unified Platform L7|ESP, with its Workflow Orchestration and Data Contextualization, meets today’s and tomorrow’s organizational needs of any life science organization aiming to digitalize their data and scientific processes to maximize the total return on information technology investment. The L7|ESP data and workflow orchestration system automates, optimizes, and executes tasks, provides a holistic approach to scientific process and business management, and can be extended with trending and charting tools for real-time operational and scientific insight.
L7|ESP creates visibility into the product life cycle process across the entire organization, from research to development, scale-up, and manufacturing or diagnostics.
An Architecture for Digitalization Resulting in Shortened Product Life Cycles
The L7|ESP platform integrates all common data information management systems, including L7 Notebooks, L7 LIMS, L7 MES, and L7 Scheduling, but also other resources such as Instrument Connectors, Sample Registration, Location, Receipt Accessioning, Logistics, Inventory, Environment Monitoring, Stability Testing, Custody of Chain, and Data Analytics.
- Works with legacy systems, allowing businesses to layer in L7|ESP alongside while continuing to use legacy systems already invested in.
- Contextualizes data across disparate legacy systems and brings them under the umbrella of a unified platform and data layer.
- Replaces redundant systems and upgrades legacy technologies, allowing the gradual decommissioning of redundant systems, while also replacing existing LIMS and ELN systems that cannot handle the emerging scientific and technology advancements.
- Ensures efficient operations, allowing the optimization of enterprise-wide scientific data and process management systems which results in optimized end-to-end (E2E) data and processes.
- Creates visibility into the product life cycle process across the entire organization from research to development, scale-up, and manufacturing or diagnostics.
- Ensures high performance in terms of cost management and insights delivery, which results in increased quality and speed of process execution.
- Creates transparency, enabling scientific information sharing between labs and programs and across the organization.
- Provides visibility on product data across the product value chain within an organization.
L7|ESP, A UNIFIED PLATFORM that contextualizes data and ELIMINATES business SILOS VIA process ORCHESTRATION
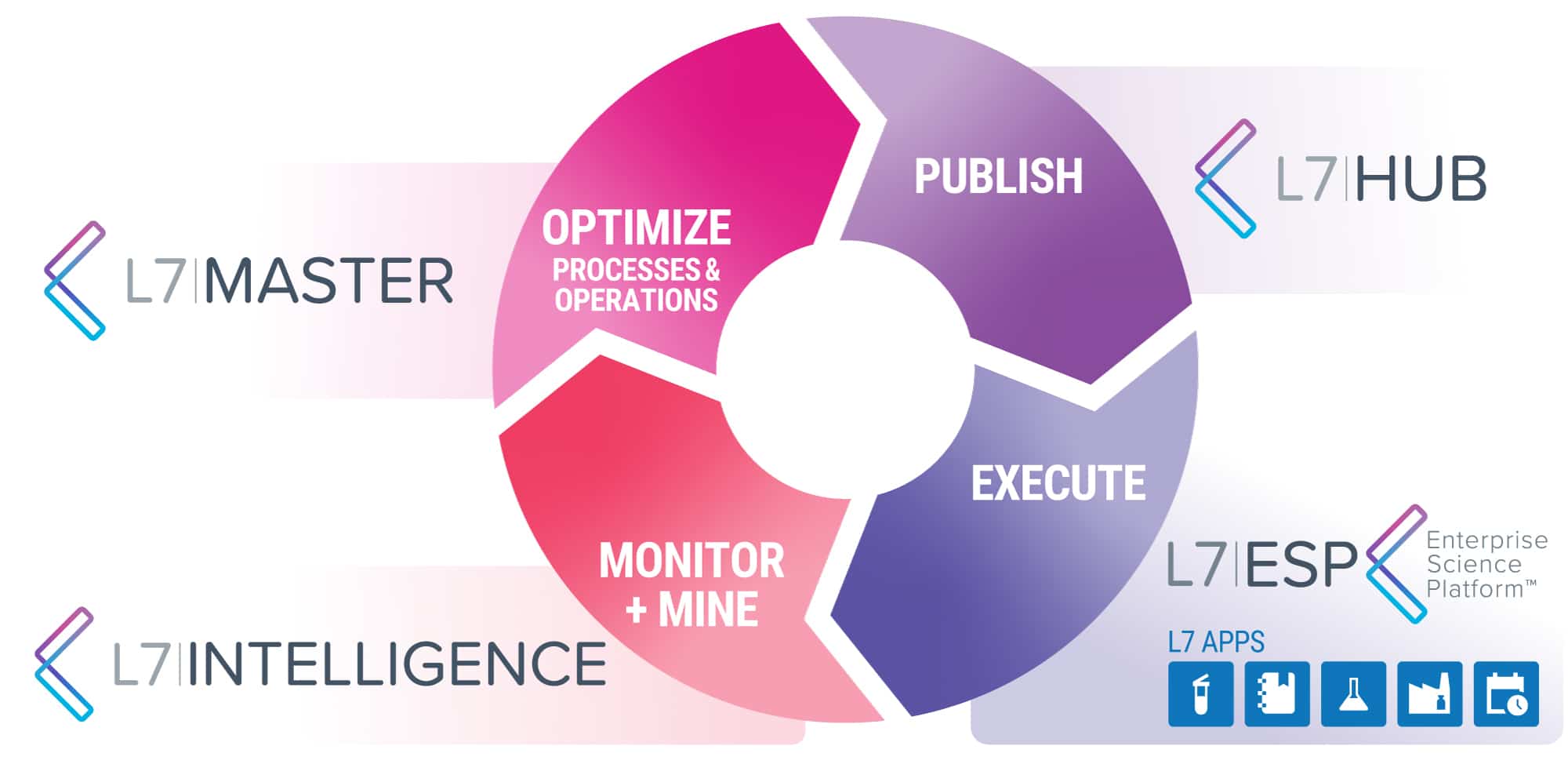
The L7|ESP’s virtuous digitalization lifecycle of designing, publishing, executing, monitoring, mining, and optimizing results in improved and redesigned processes and operations with increased operational efficiency and overall reduced costs across research, development, manufacturing, and quality operations.

L7|ESP addresses data and laboratory management needs and challenges in life sciences across the value chain, specifically in:
- Research (academia and pharma) and diagnostics: Research scientists, bioinformaticians / computational scientists, lab technicians, lab managers, and IT infrastructure managers.
- Pharma and CDMO – small and large molecules and advanced therapeutics: Process development engineers and analytical development scientists, bioinformatician/computational scientists, IT infrastructure managers, GxP experts and quality reviewers, QC/QA associates and head of QC/QA, and project/program managers
- Clinical sample management: Regulatory scientists, QC/QA associates, project/program manager
FAIRification of process and data along with the L7|ESP Process Orchestration Platform improves business intelligence, via data integration and contextualization, increases business velocity, and reduces costs.
