L7 | Contract Development and Manufacturing Organizations
data+intelligence for CDMO
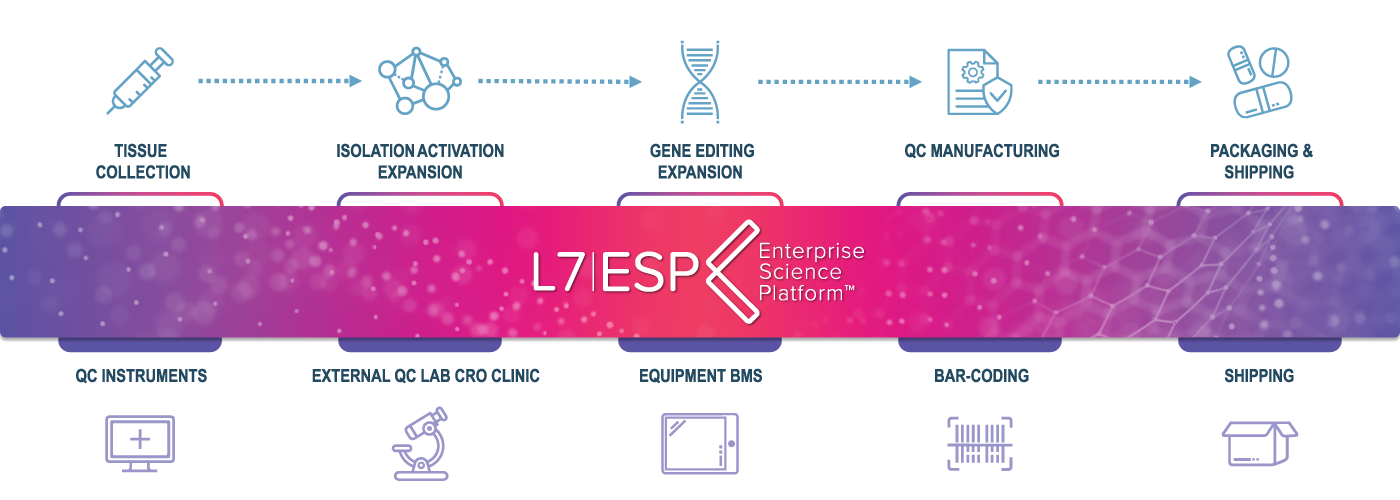
L7|ESP offers an end-to-end solution for the regulatory compliant automation of biologics manufacturing and quality control operations, from original source samples to final drug products, digitally transforming the information technology landscape.
Siloed multi-vendor IT systems make it challenging to create an integrated and validated environment for biologics manufacturing with processes involving a mixture of paper, software systems, instruments, environmental monitoring systems, and bioprocess equipment. L7|ESP is a unique software package that provides an integrated data platform for the digital transformation of the sample to drug process with features including QC LIMS, Environmental Monitoring, Electronic Batch Records (EBR), MES, CPV, inventory, sample management, equipment integration, analytics/statistical processing, and business intelligence dashboards.
L7 CDMO workflow
business benefits
VELOCITY
-
accelerate sample-to-drug
EFFICIENCY
-
easy to implement and user-driven
-
no rip and replace of existing systems
COMPLIANCE
-
CFR part11/GCP/GMP
solution capabilities
INTEGRITY
-
single data and process automation platform
-
integrated and validated environment
-
strengthen the understanding between process, product, and patient outcomes
COLLABORATION
- better collaboration between all stakeholders
CONNECTIVITY
-
software
-
environmental monitoring
-
bio-process equipment
-
quality control instrumentation


