L7 | CHATS
product highlight
Flexible and Fully Functional L7 LIMS, Integrated into the Unified Platform L7|ESP™ with Workflow Orchestration
by Brigitte Ganter | posted on January 16, 2024
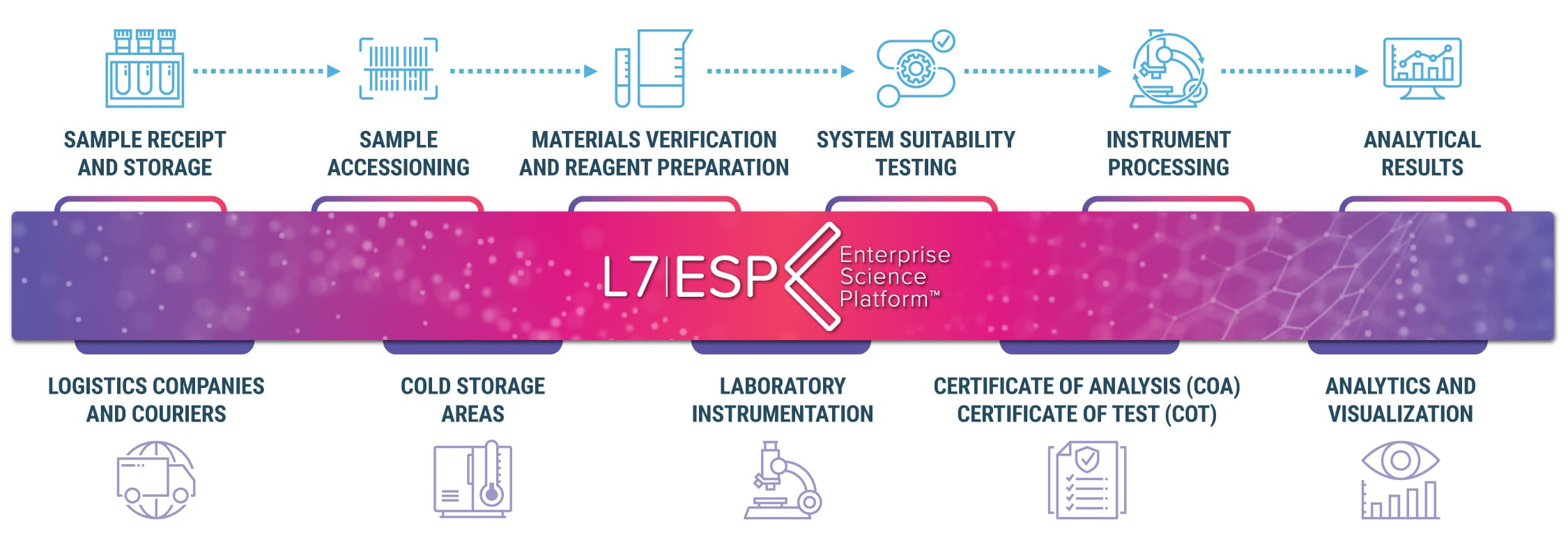
CURRENT CHALLENGES
Advances in equipment capabilities, such as high-throughput and high-frequency measurement technologies combined with innovative, new technological advancements and complex drug modalities in the therapeutics sector, have drastically changed the scale and complexity of laboratory experiments and study protocols. The paper and Excel paradigms are incompatible with the current reality of modern research and clinical laboratories. They simply cannot scale to keep up with today’s business and scientific demands, make it almost impossible to structure data for downstream process analysis, do not connect the broader lab environment, complicate collaborations, and make historical data retrieval problematic.
The result is increased technical debt as the entire process lacks a clear view of the bigger picture, such as organizational strategy; digitizing documents; automating processes; addressing and controlling data and process explosion; securing information access; minimizing information silos; connecting and integrating older legacy systems, instruments, and equipment; managing resources; managing year-over-year cost; eliminating low-quality data; and data contextualization, reporting, and analytics (AI/ML).
L7 LIMS Provides Endless Capabilities Using a Workflow Orchestration and Entity-Based Master Data Approach
While the challenges are substantial, the L7 LIMS app provides an effective and clear solution to address the many challenges life sciences research organizations face by streamlining and optimizing laboratory operations, the result of which is a highly efficient and accurate process at a reduced cost.
Key L7 LIMS benefits include:
- Track and manage samples from collection to disposal, including location, storage condition, and testing history, which results in reduced sample mix-ups, error-prone tracking, and increased traceability.
- Make data, results, protocols, and documentation available to all lab members, the entire organization, and collaborators via a centralized data repository that organizes all information, is fully searchable, and improves transparency.
- Automate and optimize workflows and tasks, which results in higher efficiency, accelerated processes, and fewer manual errors.
- Manage and optimize all your lab resources (e.g., consumables, equipment, and personnel), which results in better resource allocation and prevents shortages.
- Maintain data integrity with role-based user access controls, audit trails, and electronic signatures.
L7 LIMS Capabilities Are Fully Integrated Within L7|ESP™
- Is part of the L7|ESP Unified Platform with its Workflow Orchestration system and Data Contextualization.
- Dynamically and automatically links entries to L7|ESP data sources.
- Is a flexible, secure, and collaborative digital and process-oriented solution.
- Enforces standard operating procedures (SOPs) and regulatory guidelines.
- Generates customizable, white-label reports summarizing experimental outcomes, trends, and performance metrics.
- Follows and supports FAIR data principles.
- Is a low-code/no-code authoring tool for any scientific process.
- Easily scales and expands to any processing need.
- Is easy to navigate with a modern UI, which supports user-configured experiences.
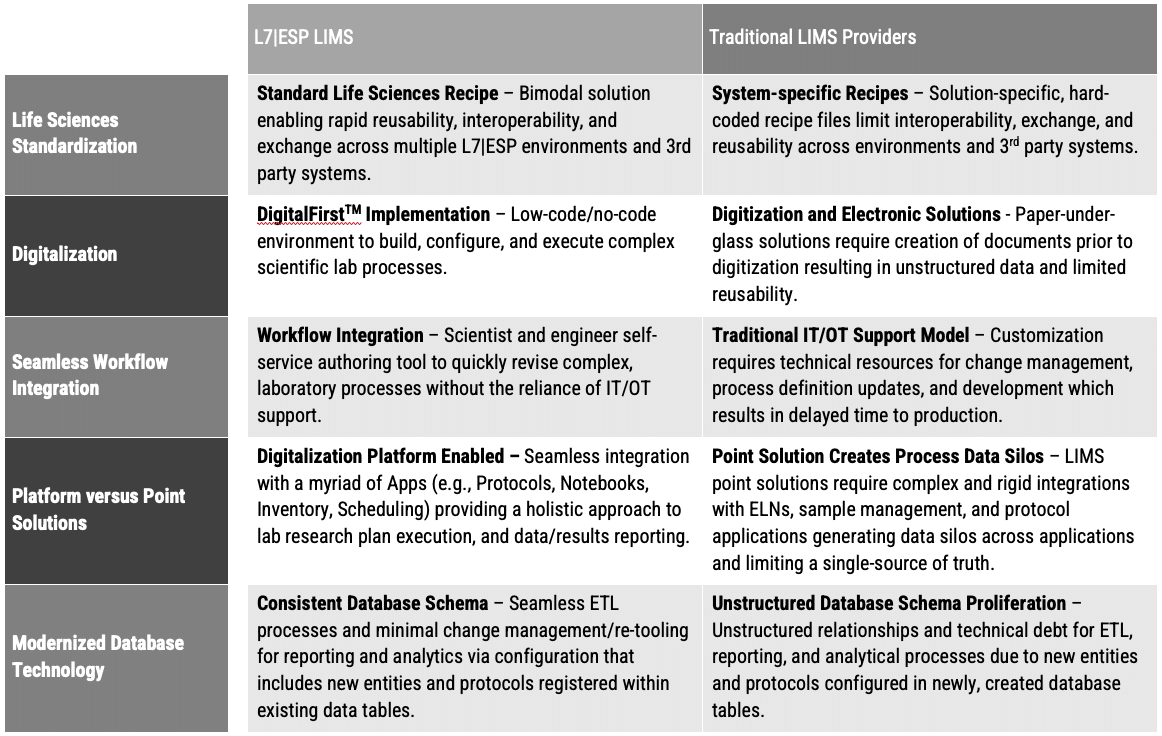
L7 LIMS Outperforms Traditional LIMS Systems
L7 LIMS is for Research Scientists and Managers
The L7 LIMS App is for researchers and operational end users across research, therapeutics, diagnostics, and AgBio. Specifically,
- Research/R&D scientists and managers in drug discovery, process development, and analytical development.
- Operational end-users which include lab and R&D technicians, bench researchers, process development engineers and analytical development scientists, lab managers, project/program managers, and GxP expert/quality reviewers.
By utilizing the flexible L7 LIMS as part of the L7|ESP Unified Platform, researchers benefit from a seamless integration of structured and unstructured data which is what most experimental paradigms require. Data is organized and accessible, follows FAIR data principles, with LIMS capabilities functioning on the operational side of the process which includes many different apps/modules.
L7 LIMS Is Part of the L7|ESP Unified Platform
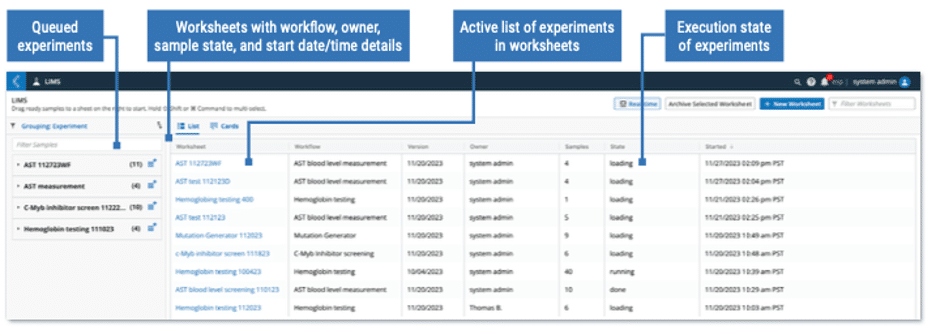
The L7 LIMS app is part of the L7|ESP Unified Platform where samples are recorded, experiments are executed, inventory is tracked and where protocols and workflows reside in the same database as Notebooks, inventory data, scheduling tools, and a myriad of other applications. The system uses existing workflow chain configurations to automate task orchestration and therefore provides a holistic approach to lab process management.
The L7 LIMS app offering, fully integrated into the Unified L7|ESP Platform with its Workflow Orchestration system, is the ideal solution for us to modernize, adapt, and improve our internal Excel spreadsheet-based processes to formalize our SOPs and to manage all entities and data in one single source of truth.
