L7 | CHATS
thought leadership
Top 5 Considerations for Lab Digital Transformation: What Gartner’s Market Convergence Means for Your Data Migration Strategy
by Teodor Leahu | posted on July 02, 2025
Here’s a question that keeps CIOs awake at night: “We have 15 years of critical laboratory data across seven different systems. How do we modernize without losing everything we’ve built?”
It’s not just about the data itself. It’s about the context, the relationships, the workflows that make that data meaningful. And increasingly, it’s about preparing for a future where laboratory informatics and business systems don’t just coexist; they converge into unified digital platforms.
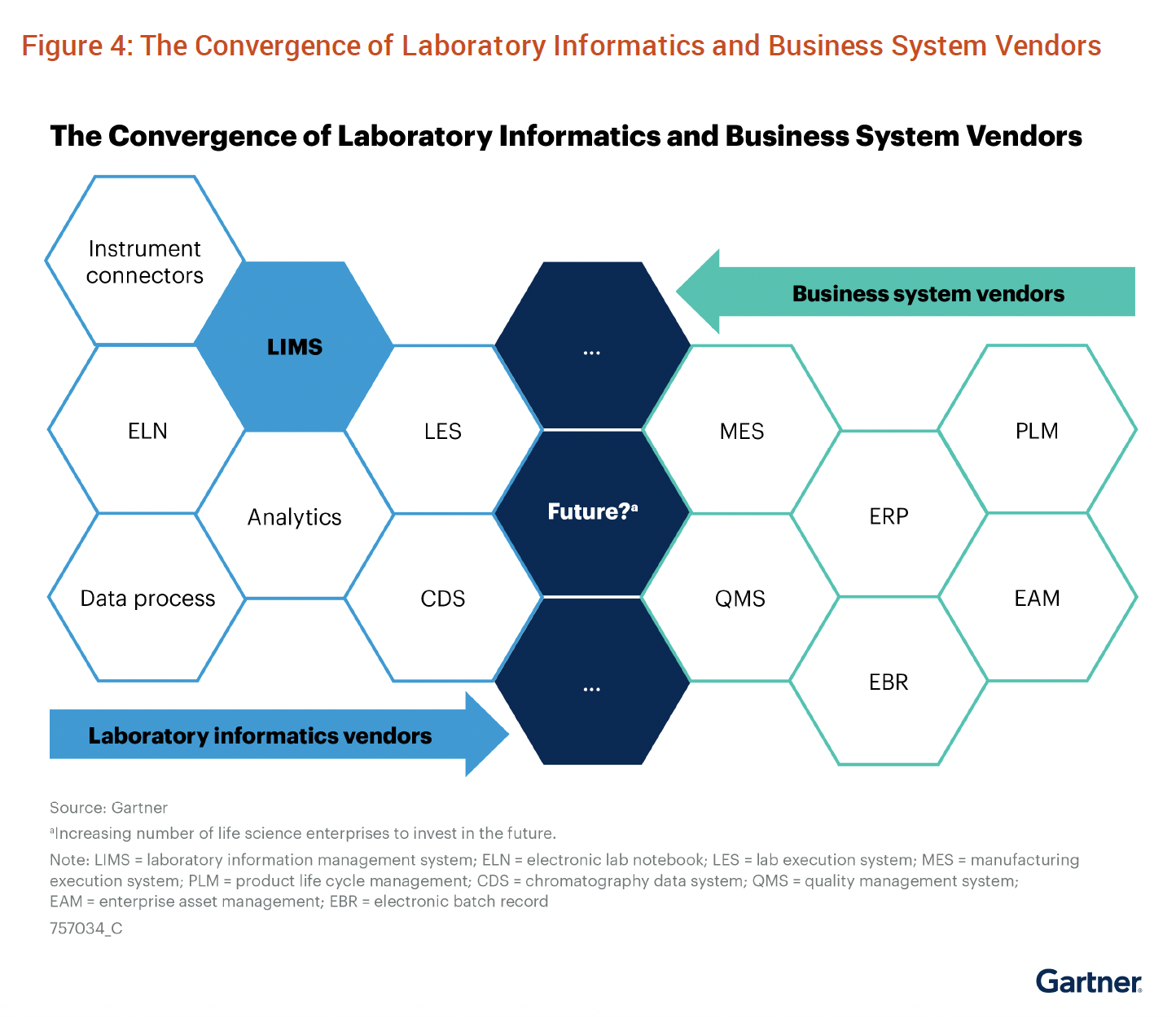
Gartner’s latest Market Guide for Laboratory Information Management Systems reveals a fundamental shift happening in our industry. The research shows that laboratory informatics vendors and business system vendors are on what they call a “collision course,” with unified platforms emerging as the new standard for enterprise-scale operations.
However, here’s what the high-level strategic guidance doesn’t fully address: how do you transition from fragmented legacy systems to a unified future without disrupting what’s already working?
The answer lies in understanding that data migration isn’t just a technical challenge; it’s the foundation of your entire digital transformation strategy.
The Convergence Challenge: Breaking Free from Vendor-Defined Constraints
Gartner’s research identifies nearly 240 LIMS vendors serving diverse market segments, each with “highly variable capabilities,” creating a “complex, hard-to-navigate set of vendors.” However, the complexity isn’t in choosing between vendors. It’s in understanding that the entire category is evolving while traditional best-of-breed approaches force you to adapt your science to vendor-defined constraints.
The traditional approach has been to use “best-of-breed solutions,” meaning selecting the best LIMS, ELN, and MES, and then attempting to integrate them. But when you select multiple point solutions, you’re not just buying software. You’re accepting that your comprehensive scientific processes will be artificially fragmented across systems that weren’t designed to work together. Your analytical workflows get squeezed into LIMS configurations. Your manufacturing execution gets separated from quality decisions.
As Gartner shows, we’re moving toward “unified laboratory informatics platforms” that bridge the gap between lab operations and business systems. This convergence creates both opportunity and challenge for data migration. The opportunity: You can finally create that single source of truth across your entire operation. The challenge: Your migration strategy needs to account for not just where your data is today, but where the entire industry is heading.
Most organizations approach data migration as a one-time event. Move from System A to System B, validate the transfer, and move on. But in the converged world that Gartner describes, migration becomes orchestration, creating dynamic connections between systems that reflect how your science actually works, not how vendors think it should work.
Five Strategic Considerations for Laboratory Digital Transformation
1. Platform Composability: Can Your Migration Strategy Adapt as Technology Evolves?
Gartner highlights a critical shift: “accelerated technological innovation has shifted the market toward composable platforming.” Traditional migration moves data from one static system to another. Composable platforms orchestrate that data across an expanding ecosystem without requiring re-migration.
How L7|ESP addresses this: The Enterprise Science Platform utilizes “flexible data modeling,” a composable architecture that enables you to define relationships between data objects regardless of their source system. When you migrate CHO cell line data from your legacy LIMS, it doesn’t just land in a new database; it becomes part of a unified data model that simultaneously supports LIMS workflows, ELN documentation, MES batch records, and business system integrations.
2. Cross-System Orchestration: Beyond Integration to Workflow Unification
Gartner notes that LIMS vendors are “advancing their interoperability and integration into other business systems, such as CRM for B2B interfacing, and ERP for supply chain cost modeling.” But integration and orchestration are fundamentally different approaches. Integration connects systems at the data level. Orchestration connects them at the workflow level… and eliminates the biggest hidden cost in laboratory digital transformation: perpetual reconfiguration.
Here’s the reality most organizations face: “I’ve spent millions configuring my analytical workflows into LIMS Vendor A. How many years and millions will it take to repeat this process for LIMS Vendor B?” The traditional answer has been “forever,” resulting in vendor lock-in due to the sheer investment in configuration.
How L7|ESP addresses this: Our unified platform approach breaks this cycle. Your workflows live in L7|ESP as executable processes that orchestrate across all your systems. Instead of rebuilding configurations every time you switch vendors, you migrate once into a platform designed for your long-term operational evolution.
3. AI-Ready Data Architecture: Is Your Migration Strategy Preparing for Intelligent Operations?
Gartner emphasizes that LIMS vendors are offering “novel technology differentiators, such as generative pretrained transformer (GPT) for searching for data such as reagents, research findings, and test results.” But AI capabilities are only as good as the data architecture they’re built on.
The most important requirement is data context. When migrating laboratory data, you’re moving scientific context, experimental conditions, equipment calibrations, and environmental factors that make those numbers meaningful.
How L7|ESP addresses this: Our platform includes “contextual data capture,” meaning every piece of migrated data retains its full scientific and operational context. When you migrate chromatography results, L7|ESP captures not just the peak areas and retention times, but the instrument conditions, method parameters, operator details, and sample preparation history. This contextualized data serves as the foundation for AI capabilities such as predictive analytics and automated anomaly detection.
4. Regulatory Compliance and Data Integrity: Beyond 21 CFR Part 11 to Comprehensive Audit Trails
Gartner’s research states “validation and regulatory compliance” as a core LIMS capability, noting that “the pharmaceutical industry requires GxP compliance in manufacturing and 21 CFR Part 11 compliance in research.” As laboratory informatics platforms converge with business systems, compliance requirements become more complex.
Your migrated data needs to maintain audit trails across business system integrations. Traditional migration often breaks audit trails, losing the historical context of who created data, when it was modified, and why changes were made.
How L7|ESP addresses this: Our platform maintains “continuous audit lineage” throughout migration and beyond. When we migrate your legacy data, every record retains its complete audit history. As that data moves through new workflows in L7|ESP, the audit trail continues seamlessly, ensuring regulatory compliance across your entire product lifecycle. And when a regulatory inspector asks about a three-year-old batch record, you can trace every decision across the entire product lifecycle.
5. Total Cost of Ownership: The Hidden Economics of Platform Convergence
Gartner shows that life science organizations are moving toward “unified platforms” away from “individual siloed best-of-breed lab informatics integration programs.” The economic implications extend far beyond the costs of software licensing.
When you migrate from multiple legacy systems into a unified platform, you fundamentally change your operational economics. The value lies in operational efficiency gains from unified workflows.
How L7|ESP addresses this: Our platform delivers what Gartner calls “enterprise enablement that maximizes total ROI.” Our customers typically see 40% reductions in data management overhead and 30% improvements in process cycle times. One client reduced their batch record cycle time by six days simply by eliminating manual data transfers between their LIMS, ELN, and MES systems.
The Strategic Imperative: Why Platform Convergence Can’t Wait
Gartner’s research concludes: “Unified laboratory informatics platforms are on a collision course with business systems as the demand for end-to-end workflows emerges as a must-have capability.” This convergence isn’t a future possibility. It’s happening now.
The companies that will thrive are those that recognize data migration as the foundation of platform transformation. When you approach migration strategically, with composable architecture, cross-system orchestration, AI-ready data models, comprehensive compliance, and total economic impact in mind, you’re not just solving today’s data challenges; you’re positioning your organization for the converged future that Gartner describes.
From Migration to Orchestration: The L7|ESP Advantage
L7|ESP represents exactly this convergence. We’re not just a LIMS, ELN, or MES. We’re a unified Enterprise Science Platform that orchestrates data and workflows across the entire spectrum of laboratory and business operations. When you migrate to L7|ESP, you’re not just moving data; you’re transforming how your organization operates.
The question isn’t whether your industry will embrace platform convergence. Gartner’s research makes clear that it will. The question is whether your organization will lead that transformation or be forced to catch up.
Your data migration strategy is your digital transformation strategy. Make sure it’s designed for the converged future, not just the fragmented past.
Contact us to start the conversation about your digital transformation journey.
___________
Source:
Gartner Research: Michael Shanler, Reuben Harwood, Lynn Rice, Market Guide for Laboratory Information Management Systems (16 April 2025)
