L7 | CHATS
case study
Creating an Efficient and Transparent Laboratory and Data Management Process to Expedite the Formalization of Internal R&D Standard Operating Procedures (SOPs)
by Brigitte Ganter, Ph.D. | posted on June 05, 2024
Developing new medicines for traditionally difficult protein targets requires new approaches that can easily be compared to building an airplane from scratch and learning to fly it. Think Bioscience is exactly at this stage. They are utilizing synthetic biology to both identify new natural products that can act as starting points for new therapeutic development and also help screen through those newly identified natural products in an efficient way. Think Bioscience’s approach is unique, in that they are optimizing their entire laboratory and data management processes (see Figure 1) while also formalizing their internal R&D Standard Operating Procedure (SOP). In other words, they are trying to fly the plane while still building it.
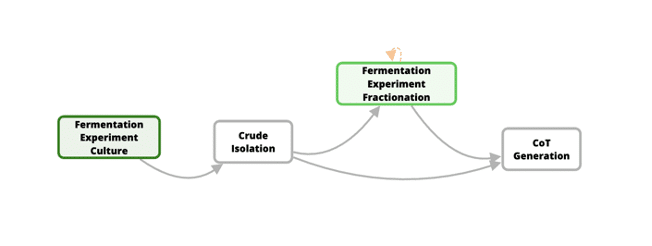
Figure 1: High-level description of Think Bioscience’s approach to optimizing their entire laboratory and data management processes.
To scale and standardize their proof-of-concept (PoC) processes and ensure all data collected follows a rigorously established SOP, all disparate data points (including metadata) are tracked and linked back to each specific experiment available within a centralized database. Think Bioscience needed to modernize, adapt, and improve their internal Excel spreadsheet-based processes to accommodate their need to manage all entities and data in one single source of truth. The Unified Platform L7|ESP®, with its Workflow Orchestration system, was identified as the perfect solution to bring organization, efficiency, and scalability to this process while formalizing the internal SOP process. Furthermore, to identify new protein targets using plasmid libraries and ensure efficient screening and identification of sequences of interest, and to be able to search through existing libraries in bulk, Think Bioscience took advantage of the plasmid editor (see Figure 2), data ingest app, and search capabilities available as open-source components in the L7|HUB to create an efficient and transparent process.
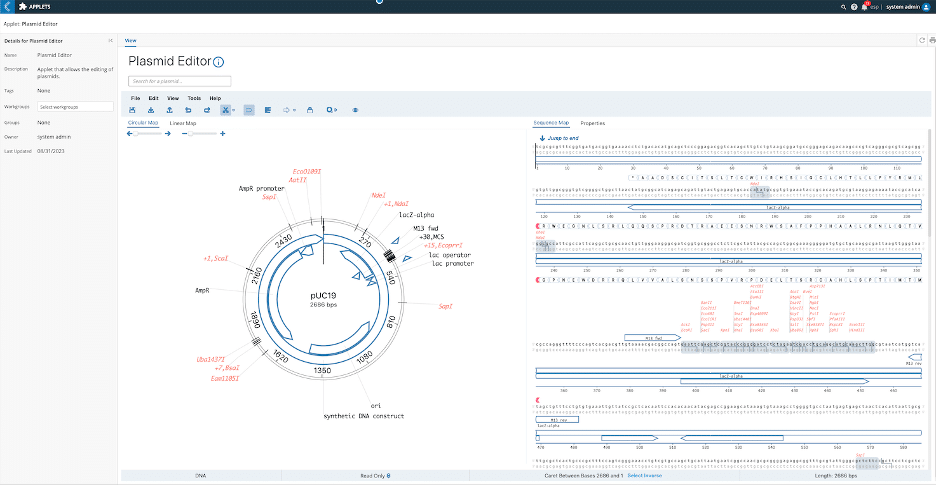
Figure2: Plasmid editor available as an open-source component in the L7|HUB.
L7|ESP Is the Solution to Overcome their Challenges
| CHALLENGES | L7|ESP PROVIDES THE SOLUTION |
|
Tracking and collecting large disparate data at scale
|
|
|
Formalizing SOPs While Still Developing the Experimental Details
|
|
| Understanding Process Efficiencies and Inefficiencies |
|
| Managing Data Integrity Risk |
|
| Maintaining all Plasmid Data |
|
| Efficiently Creating and Managing the Many Relationships Between DNA and Chemical Products |
|
| Scaling the PoC |
|
| Ensuring Adherence to Data Provenance |
|
L7|ESP Value-Add
The Unified Platform, L7|ESP, with its Workflow Orchestration system, brings organization, efficiency, and transparency to Think Bioscience’s R&D SOP implementation process, facilitating disparate data collection, tracking, and storing. L7|ESP also streamlines library screening and data generation, integration of a plasmid editor, plasmid libraries with associated metadata, and efficient data mining and retrieval of its organizational data hub. The system not only allows for better data tracking, but also enables scalability, transparency, and connectivity between team members involved in rewriting SOPs to formalize internal processes. In other words, L7|ESP is helping Think Bioscience fly faster.
The Unified L7|ESP Platform, with its Workflow Orchestration system, is the ideal solution for us to modernize, adapt, and improve our internal excel spreadsheet-based processes to formalize our SOPs and to manage all entities and data in one single source of truth.
