L7 | CHATS
thought leadership
Beyond the Limits of LIMS: Why Life Sciences Needs a Unifying Platform Approach
by Will Gray | posted on April 08, 2025
For decades, life sciences organizations have relied on an expanding array of point solutions to manage laboratory operations. Nowhere is this more evident than in the LIMS (Laboratory Information Management System) market, where vendors continue to multiply, each promising better data management, workflow automation, and regulatory compliance. With the global LIMS market projected to grow from $2.4 billion in 2025 to $4.6 billion by 2032, the demand for these solutions remains strong.
Yet, despite this rapid expansion, life sciences organizations still grapple with fundamental challenges: data silos, disconnected workflows, and an increasingly complex IT landscape. The question is no longer whether LIMS is necessary but whether traditional LIMS solutions alone can meet the evolving needs of modern research, development, and manufacturing.
The answer? They can’t. The future lies in a unifying platform approach—one that goes beyond LIMS to orchestrate processes, contextualize data, and ensure seamless integration across the entire enterprise.
The Pitfalls of Legacy LIMS: Myths vs. Reality
LIMS has long been viewed as the cornerstone of lab operations, but traditional solutions have inherent limitations that hinder their ability to support enterprise-wide digital transformation.
▶ Myth #1: LIMS is a comprehensive solution for lab data management
Reality: LIMS was originally designed for one primary function—managing assay results for quality control and sample testing. Over time, vendors have bolted on additional features like lab execution modules (LES) and lightweight electronic lab notebooks (ELN), but these additions remain rigid and fail to support end-to-end workflow execution.
▶ Myth #2: LIMS scales effortlessly with an organization’s needs
Reality: Legacy LIMS are often static, built on outdated architectures that don’t scale with evolving workflows. Many organizations find themselves managing multiple LIMS across different sites and departments, leading to a fragmented system landscape rather than a scalable solution.
▶ Myth #3: LIMS integrates seamlessly with other lab systems
Reality: The integration of LIMS with ELN, MES (Manufacturing Execution Systems), and material management systems is complex, costly, and often unreliable. Many legacy LIMS struggle to communicate with laboratory instruments, requiring manual data transfers that increase errors and compliance risks.
The Harsh Reality of Integrating Point Solutions
Recognizing the limitations of standalone LIMS, many organizations have attempted to patch together multiple-point solutions. In theory, this should create a more comprehensive lab ecosystem. In practice, it introduces a host of new challenges:
- Complex Integrations: Each new system added increases IT complexity, making system maintenance and upgrades a nightmare.
- High Maintenance Costs: Integrating multiple systems requires constant updates, troubleshooting, and expensive middleware solutions.
- Data Fragmentation: Data is scattered across multiple systems, making it difficult to achieve a single source of truth.
- Compliance Risks: When critical data is spread across disparate systems, tracking contamination events, ensuring regulatory adherence, and maintaining audit trails become significantly more challenging.
One life sciences executive recently shared how their organization believed they had achieved end-to-end automation—only to later realize they had 30 different labs operating in complete isolation. This illusion of connectivity is common among companies relying on disparate systems that don’t truly communicate with each other.
A New Path Forward: The Unifying Platform Approach
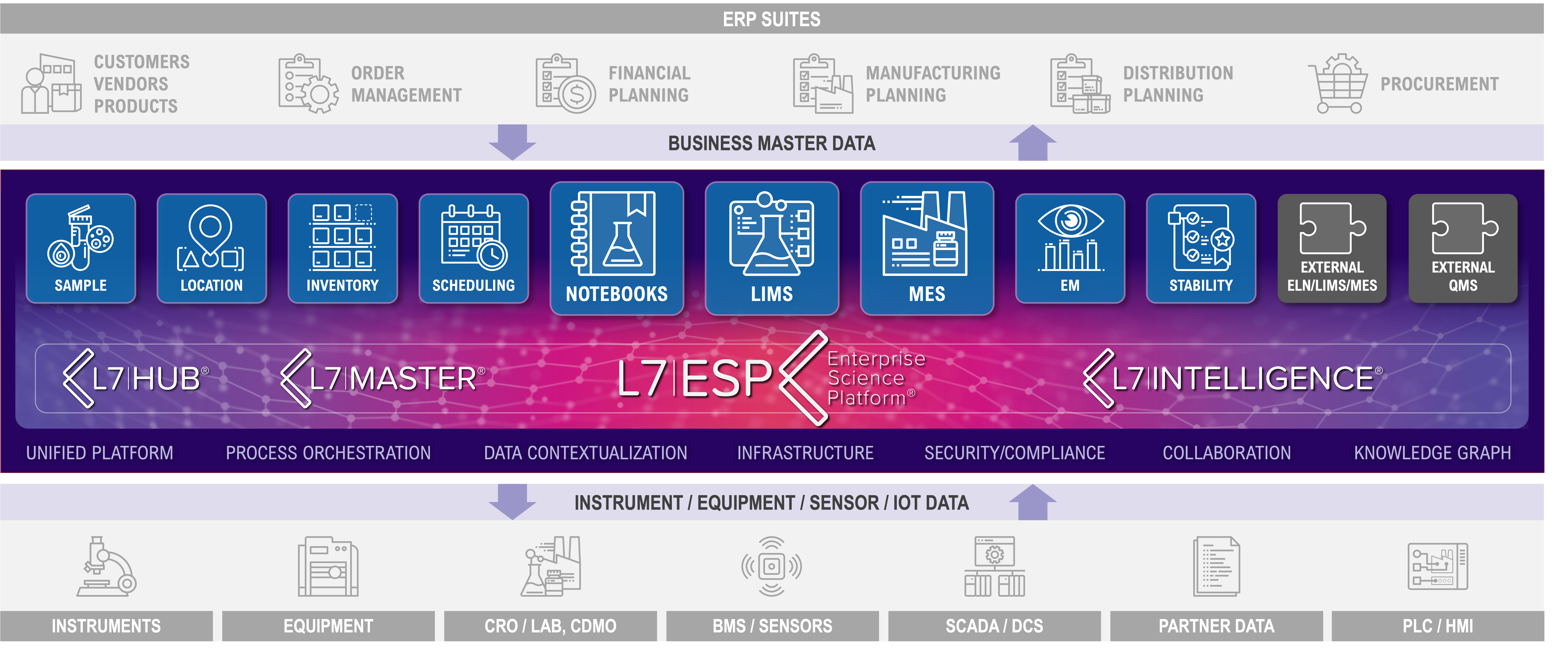
Rather than continuing to stack disconnected solutions on top of each other, forward-thinking organizations are adopting a unifying platform approach. A true unifying platform does more than act as a LIMS—it orchestrates workflows, contextualizes data, and integrates seamlessly with existing systems to eliminate silos. This is where L7|ESP® fits in.
L7|ESP is not just another LIMS—it is an enterprise-wide orchestration platform that:
- Provides an integrated ecosystem with native applications (LIMS, ELN, LES, etc.) while allowing organizations to keep third-party solutions in place if desired.
- Orchestrates workflows and contextualizes data across R&D, clinical development, and manufacturing for a true end-to-end approach.
- Enhances regulatory compliance by ensuring standardized processes, audit trails, and real-time tracking of critical data.
- Offers unmatched scalability and flexibility, allowing organizations to expand and adapt their digital landscape without the risk of vendor lock-in.
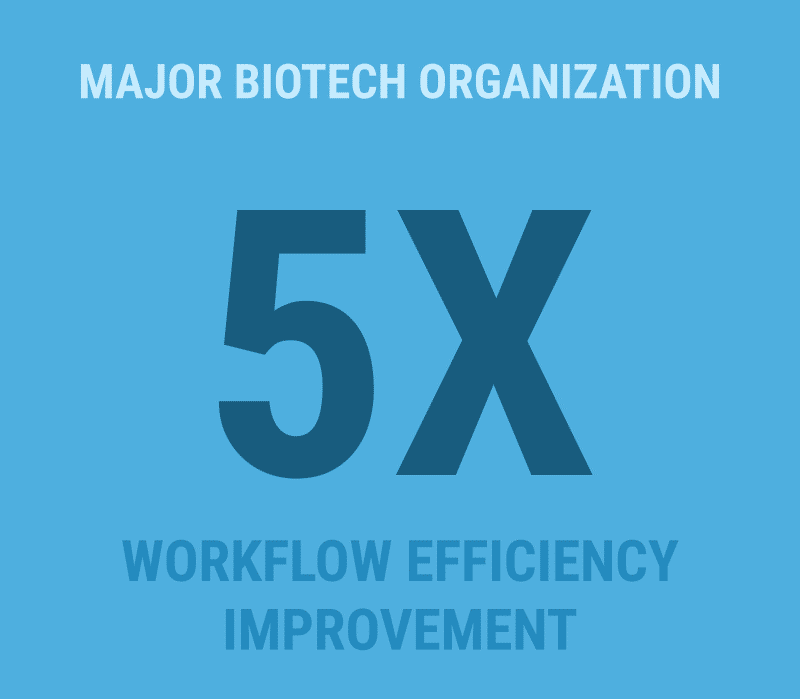
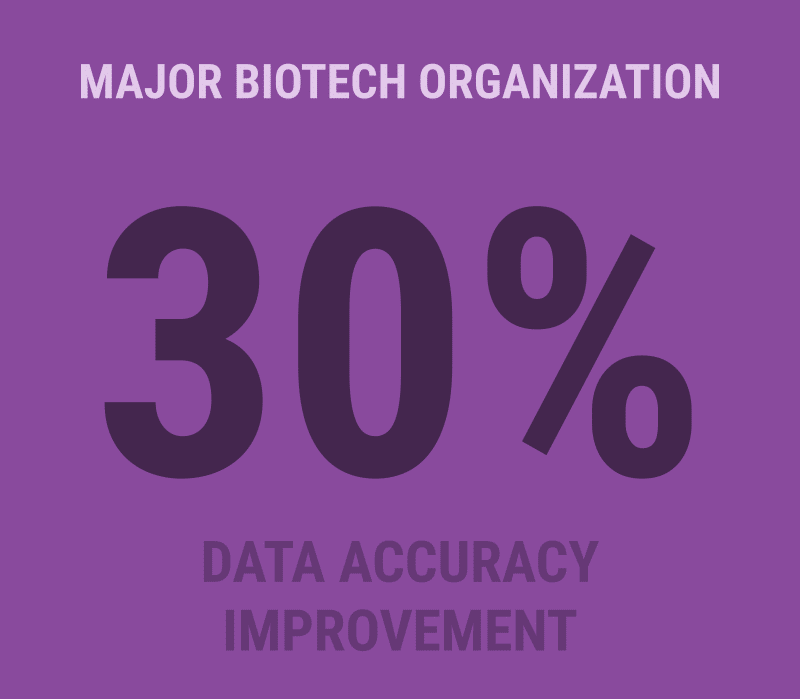
A major biotech organization recently leveraged L7|ESP to consolidate multiple disconnected systems, dramatically improving data accuracy and workflow efficiency. Another customer in the contract development and manufacturing space (CDMO) reduced time-to-market by up to 40% by shifting from a fragmented system landscape to an orchestrated platform approach.

The Bottom Line: LIMS Alone Won’t Drive Digital Transformation
Legacy LIMS solutions were never designed to handle the scale and complexity of today’s life sciences operations. The industry is rapidly moving toward AI-driven insights, automated workflows, and enterprise-wide digital transformation—initiatives that cannot succeed without a data-first approach.
Only organizations that embrace a unifying approach—one that seamlessly connects research, development, and manufacturing—will be truly AI-ready. Without this level of contextualized data and orchestration, companies risk falling behind in the race toward smarter, faster, and more efficient life sciences operations.
The days of relying on standalone LIMS are over. The future belongs to platforms that unify, orchestrate, and transform the way science is done. Contact us if you’re ready to explore L7|ESP, or visit our website at L7 Informatics.